Abstract
Even in the era of the targeted therapies, there remains clinical value in exploring the impact of disease characteristics in chemo-immunotherapy (CIT) trials, namely to identify features that contribute most to long-term outcomes, thereby pinpointing patients destined to benefit from these therapies. One such feature is the CLL DNA methylome, that recapitulates normal B cell maturation, with IGHV mutated (M-CLL) and unmutated CLL (U-CLL) retaining an imprint of the DNA methylation signature of memory (m-CLL) and naive B cells (n-CLL), respectively, with a third intermediate epigenetic subgroup (i-CLL) with borderline mutation status. The pyrosequencing analysis of 5 CpG sites can divide CLL into these three subgroups (Queiros 2015, Leukemia 29:598-605), each with different clinico-biological features.
We classified 606 treatment-naïve patients randomized to one chemotherapy (LRF CLL4) and two CIT (ARCTIC and ADMIRE) trials, into the three epigenetic subgroups using pyrosequencing and confirmatory Infinium HumanMethylation450 analysis (n=60, 96% concordance between technologies). Associations between epigenetic and clinico-biological features, progression-free (PFS) and overall survival (OS) were identified by linear regression, univariate Kaplan-Meier and multivariate Cox regression analysis.
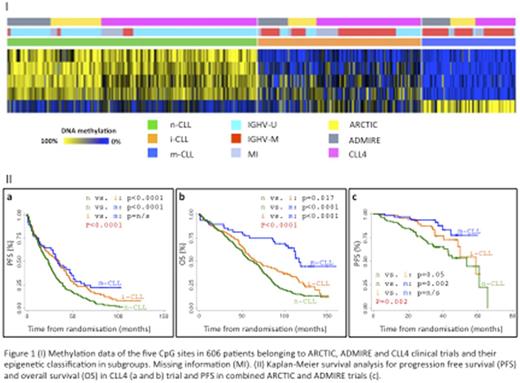
We identified n-, i- and m-CLL in 49.3% (n=299), 32.0% (n=195) and 18.5% (n=112) of our patients, respectively (Fig1I). Fewer m-CLL patients were identified in our study compared to published data, reflecting the progressive nature of our cohort, with 80% (n=245/305, P<0.001) of U-CLL cases exhibited the n-CLL signature (i-CLL: 17% and m-CLL: 3%). For M-CLL cases 9%, 50% and 41% exhibited the n-, i- and m-CLL epigenetic signature, respectively. Median IGHV mutation load differed between epigenetic subgroups (p<0.001), with n-, i- and m-CLL displaying 100%, 96.65% and 92.90% identity to germline, respectively. Significant associations were observed with IGHV family usage, such as an enrichment of n-CLL in IGHV1-69 cases (96%, p<0.001). 74% of stereotyped subset #2 cases were i-CLL (p<0.001). 68% (80/117, p<0.001) of cases with deletion of 11q, 77% (41/53, p<0.001) with trisomy 12 and 68% (38/56, p=0.035) with mutation and/or deletion of the TP53 gene were n-CLL. Cases with mutations in NOTCH1 (including the 3' UTR, p=0.01) and SF3B1 (p=0.02) were also enriched in n-CLL.
In 359 CLL4 patients, m-CLL was more prevalent in patients with a good response to therapy (55%, CR) compared to poor responders (22%, NR/PD). All four deaths from Richters transformation were n-CLL. Ten year OS differed according to epigenetic signature (P<0.001) and was reached by only 14% of n-CLL patients. n-, i- and m-CLL patients exhibited a median PFS of 23, 34 and 35, and OS of 63, 66 and 106 months, respectively. n-CLL showed significantly shorter PFS than i-CLL (HR 0.64, p<0.001) and m-CLL (HR 0.52, p<0.001), and had the shortest OS, again compared to i-CLL (HR 0.73, p=0.01) and m-CLL (HR 0.33, p<0.001, Fig1IIa and b). Multivariate Cox proportional analysis, controlling for confounding variables (inc. clinical features, IGHVstatus, TP53, NOTCH1 and SF3B1) in 278 patients showed that m-CLL was an independent prognostic factor for OS (HR 0.46, p<0.01), but not PFS.
In 247 patients randomized to ARCTIC and ADMIRE, the epigenetic signature was associated with MRD status (p=0.04), with 61% (38/62) of MRD negative patients showing m-CLL. Seventy eight percent (43/55) of m-CLL patients achieved a complete remission. In univariate analysis, the i- (HR:0.57, p=0.05) and m-CLL (HR:0.3, p=0.002) subgroups displayed longer PFS (Fig1Ic). In a multivariate model, including TP53 lesions and IGHV status (239 patients), the m-CLL subgroup retained independent prognostic significance (HR:0.25, p<0.001).
In conclusion, we report the first analysis of the clinical utility of epigenetic subgroup signatures in patients entered into first-line chemotherapy and CIT trials. In doing so, we validate key associations between epigenetics and clinico-biological disease features. Importantly, we identify m-CLL as an independent marker of survival in both our LRF CLL4 and ARCTIC/ADMIRE cohorts, providing preliminary evidence that DNA methylation analysis may aid in the identification of patients destined to demonstrate durable remissions when treated with these agents.
Steele: Gilead: Consultancy, Honoraria; Portola Pharmaceuticals: Consultancy, Honoraria, Research Funding. Pettitt: Napp: Research Funding; GSK/Novartis: Research Funding; Gilead: Honoraria, Research Funding; Chugai: Research Funding; Celgene: Research Funding; AstraZeneca: Research Funding; Roche: Research Funding. Hillmen: Janssen: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Celgene: Research Funding; GSK: Consultancy, Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie Company: Honoraria, Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding. Schuh: Celgene: Honoraria; Gilead: Honoraria, Research Funding; Janssen: Honoraria; Abbvie: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Gilead: Consultancy; Roche: Honoraria; Roche: Honoraria; Novartis: Honoraria; Janssen: Honoraria; Abbvie: Honoraria. Strefford: Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal